SERVICES
Expert medical device project leadership and program management support, planning and coordination, driving timelines to meet goals and effective communication.
Risk Management strategies and documentation to mitigate risk and ensure compliance.
Procurement link to projects maximise value for money. Vendor selection support.
Expertise in end to end effective, pragmatic project documentation.
External project review to highlight pragmatic solutions to meet objectives
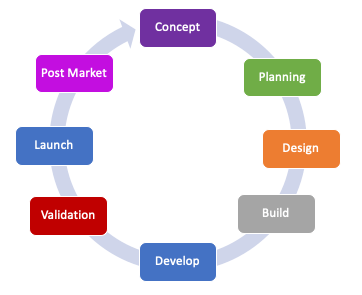
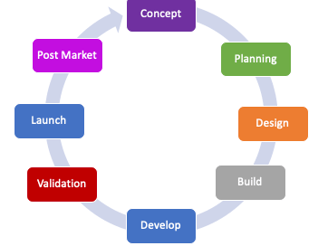
Project Management Consulting:
Industrialisation Phase Consulting:
Strategies for efficient transition from development to mass production.
Management of complex assembly line installations and tooling capacity.
Verification and validation phase plan and documentation.
Oversight of product launch readiness, including PAI audit status management.
Life Cycle Management Consulting:
Continuous improvement and standardisation strategies for existing products.
Cost reduction and sustainability strategies.
Change control governance and regulatory compliance (e.g.FDA 21 CFR 820, EU MDR 2017/745).
Technical investigation, resolution, and asset refurbishment planning.
CAPA implementation and management.
Leafry specialises in the device development and manufacture activity but has extensive experience of all phases of the lifecycle.
Guidance on R&D interface and proof of concept strategies.
Design for manufacture and robustness testing.
Assistance in achieving stability in small scale early batches.
Expertise in equipment selection, design, and approval processes.
Development road map strategy to avoid regulatory delay.
Development Phase Consulting:
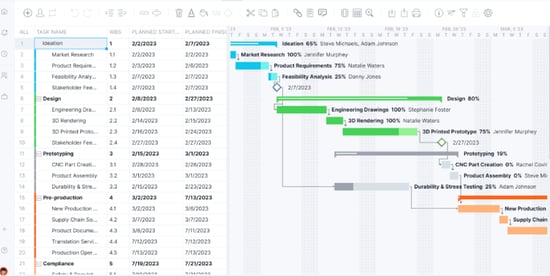
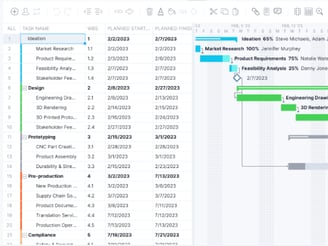
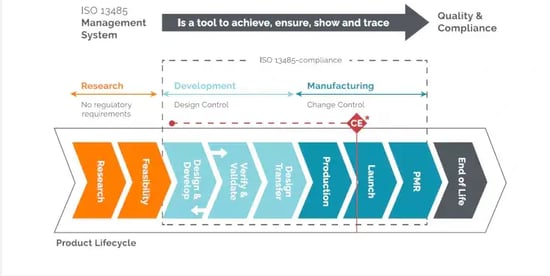
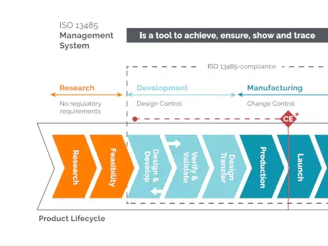
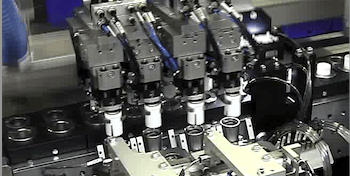
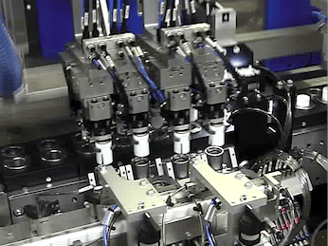
Manufacturing Equipment selection,build and validation:
Effective specification definition and sourcing strategy across pharma manufacturing equipment.
Supplier selection and management
Project, risk management.
Validation process documentation and execution.
Stuart Farley
Hitchin, Hertfordshire
Mobile - +44 (0) 7717801860
Email - sfarley@leafry.co.uk


